In 1803, London was shocked by a public experiment conducted by an Italian scientist named Giovanni Aldini—nephew of Luigi Galvani, whose experiments with electrical currents gave the term galvanism its name. Aldini acquired the body of a recently executed criminal (a perfectly legal transaction, thanks to England’s Murder Act of 1752) and applied electric stimulus to the cadaver’s face. As reported in the Newgate Calendar of that time,
…the jaws of the deceased criminal began to quiver, and the adjoining muscles were horribly contorted, and one eye was actually opened. In the subsequent part of the process the right hand was raised and clenched, and the legs and thighs were set in motion. [source]
In other words, the corpse appeared to move and blink as though it had been restored to life. This gruesome experiment, repeated at various points in time by other scientists in the early nineteenth century, made an enormous impact its spectators. Well-educated and acquainted with her father’s intellectual cohort, Mary Shelley was certainly aware of early galvanism experiments. You might perceive an echo of the Newgate Calendar account at the moment of Frankenstein when the fanatical scientist’s creation awakens violently to life:
It was on a dreary night of November that I beheld the accomplishment of my toils. With an anxiety that almost amounted to agony, I collected the instruments of life around me, that I might infuse a spark of being into the lifeless thing that lay at my feet. It was already one in the morning; the rain pattered dismally against the panes, and my candle was nearly burnt out, when, by the glimmer of the half-extinguished light, I saw the dull yellow eye of the creature open; it breathed hard, and a convulsive motion agitated its limbs. [Frankenstein Chapter 5]
Although we are all well-acquainted with the lightning bolts and sparking electrical equipment favored by cinematic adaptations of Frankenstein, Mary Shelley never specified the source of the “spark of being;” other passages in the book suggest that Victor Frankenstein’s tools were of a chemical or alchemical nature. Our curiosity about this matter brought us to Frederick T. Schaefer, Associate Professor of Chemistry and Biochemistry at the University of the Sciences. With the help of his Professor Schaefer and his team of graduate students, we learned a little bit about chemistry and electricity–and where the two connect.

For the first experiment, we asked Professor Schaefer and his assistants to help us create an electrical charge. We may not know what infused the spark of life in Frankenstein’s creature, but we know what our spark of life is: caffeine! So we headed to Saxbys Coffee and asked for some used coffee grounds.

Our intrepid scientists embedded flat pieces of zinc and copper into the coffee grounds, and clipped wires to them. According to Professor Schaefer, electrons move through the zinc metal to the copper metal by way of these attached wires, but in order for this to happen, the zinc must be in the presence of salt or acid. Acidic coffee grounds play that role here, although some other comestibles can work: powering clocks or lights from pickles, lemons, or potatoes is a popular at-home experiment.

Team Science also brought us a wonderfully gross experiment relating to Dracula. They prepared two chemical solutions: one clear, one the color of blood. When the blood-red solution was introduced to the clear solution, they two substances reacted, causing the red solution to become increasingly solid over time. Since the red solution was squeezed into its chemical bath through a dropper, the result was a long slimy thread that looks a little like… a vein?
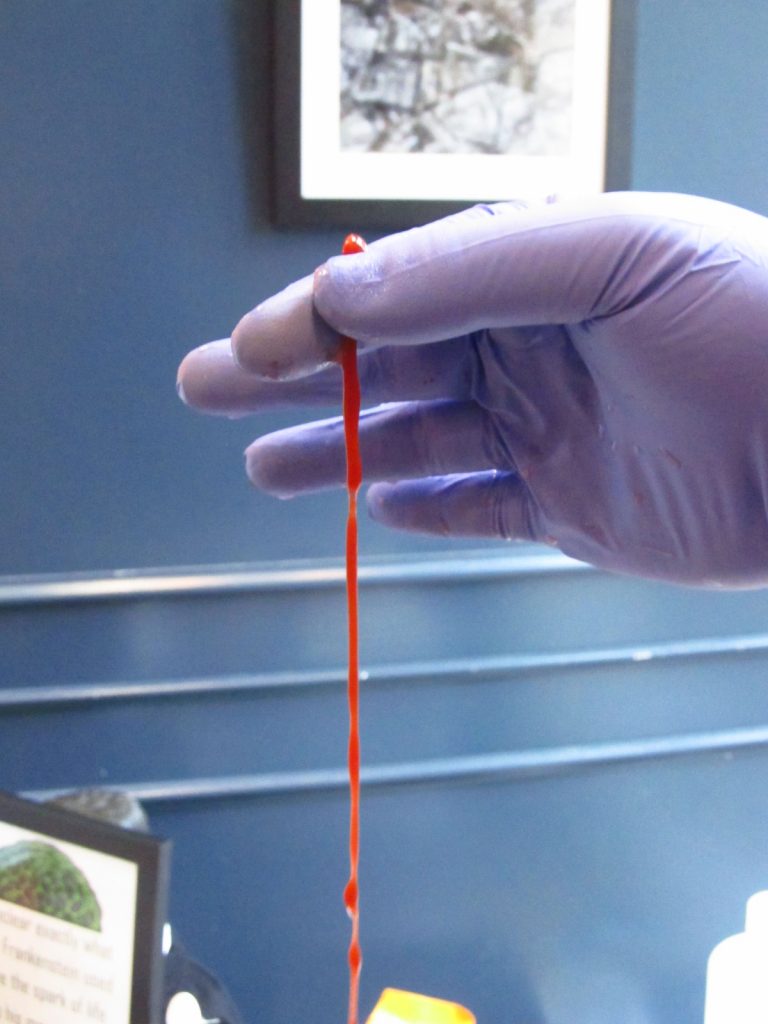
We performed these experiments several times for obliging patrons of the Saxby’s on 40th and Locust. If you missed us and are curious about the science, stop by the Saxbys at 34th and Lancaster around 2:00 p.m. on November 28 or 4:00 p.m. on December 2. We’ll show you our science, and you can pick up a brain-shaped stress reliever. These squeezable brains are more than a novelty: show yours at the front desk of the Rosenbach, and you’ll be eligible for free museum admission during the run of Frankenstein & Dracula: Gothic Monsters, Modern Science.

Our Rosenstaff has had so much fun learning chemistry from these University of the Sciences scholars. We joked about how well we got along and how much we had in common–the sciences and the humanities, together at last!–but in fact there wasn’t much of a distinction between these fields in Mary Shelley’s era, and we don’t think they are all that separate today. Featuring scientific documents as well as literary treasures, Frankenstein & Dracula explores the ways literature has been inspired by new technologies—and vice versa.
